
Synthesis of Spirocyclic Indolenines - James - 2016 - Chemistry – A European Journal - Wiley Online Library

Microstructure evolution and acid corrosion behavior of CoCrFeNiCu1−xMox high-entropy alloy coatings fabricated by coaxial direct laser deposition - ScienceDirect

Perfluoroaryl and Perfluoroheteroaryl Reagents as Emerging New Tools for Peptide Synthesis, Modification and Bioconjugation - Brittain - 2022 - Chemistry – A European Journal - Wiley Online Library

Coumarin-Caged Compounds of 1-Naphthaleneacetic Acid as Light-Responsive Controlled-Release Plant Root Stimulators | Journal of Agricultural and Food Chemistry
Stepwise recombination suppression around the mating-type locus in an ascomycete fungus with self-fertile spores | PLOS Genetics
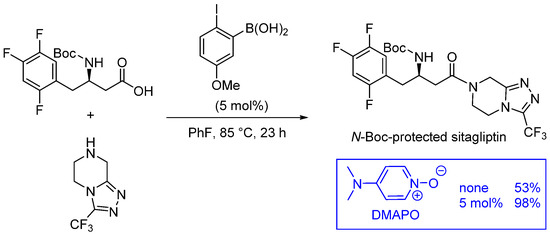
Catalysts | Free Full-Text | Direct Catalytic Amidations from Carboxylic Acid and Ester Derivatives: A Review
![Brønsted Acid-Controlled [3 + 2] Coupling Reaction of Quinone Monoacetals with Alkene Nucleophiles: A Catalytic System of Perfluorinated Acids and Hydrogen Bond Donor for the Construction of Benzofurans | The Journal of Organic Chemistry Brønsted Acid-Controlled [3 + 2] Coupling Reaction of Quinone Monoacetals with Alkene Nucleophiles: A Catalytic System of Perfluorinated Acids and Hydrogen Bond Donor for the Construction of Benzofurans | The Journal of Organic Chemistry](https://pubs.acs.org/cms/10.1021/jo400613z/asset/images/large/jo-2013-00613z_0010.jpeg)
Brønsted Acid-Controlled [3 + 2] Coupling Reaction of Quinone Monoacetals with Alkene Nucleophiles: A Catalytic System of Perfluorinated Acids and Hydrogen Bond Donor for the Construction of Benzofurans | The Journal of Organic Chemistry
Perfluoroaryl and Perfluoroheteroaryl Reagents as Emerging New Tools for Peptide Synthesis, Modification and Bioconjugation
![Brønsted Acid-Controlled [3 + 2] Coupling Reaction of Quinone Monoacetals with Alkene Nucleophiles: A Catalytic System of Perfluorinated Acids and Hydrogen Bond Donor for the Construction of Benzofurans | The Journal of Organic Chemistry Brønsted Acid-Controlled [3 + 2] Coupling Reaction of Quinone Monoacetals with Alkene Nucleophiles: A Catalytic System of Perfluorinated Acids and Hydrogen Bond Donor for the Construction of Benzofurans | The Journal of Organic Chemistry](https://pubs.acs.org/cms/10.1021/jo400613z/asset/images/jo400613z.social.jpeg_v03)
Brønsted Acid-Controlled [3 + 2] Coupling Reaction of Quinone Monoacetals with Alkene Nucleophiles: A Catalytic System of Perfluorinated Acids and Hydrogen Bond Donor for the Construction of Benzofurans | The Journal of Organic Chemistry

Lewis acid-catalyzed reactions of donor–acceptor cyclopropanes with furan derivatives - ScienceDirect

Perfluoroaryl and Perfluoroheteroaryl Reagents as Emerging New Tools for Peptide Synthesis, Modification and Bioconjugation - Brittain - 2022 - Chemistry – A European Journal - Wiley Online Library

Mechanism of Iodine(III)‐Promoted Oxidative Dearomatizing Hydroxylation of Phenols: Evidence for a Radical‐Chain Pathway - Kraszewski - 2020 - Chemistry – A European Journal - Wiley Online Library

In Situ Formation of N-Trifluoroacetoxy Succinimide (TFA-NHS): One-Pot Formation of Succinimidyl Esters, N-Trifluoroacetyl Amino Acid Succinimidyl Esters, and N-Maleoyl Amino Acid Succinimidyl Esters | The Journal of Organic Chemistry

Light-Induced Hetero-Diels−Alder Cycloaddition: A Facile and Selective Photoclick Reaction | Journal of the American Chemical Society
![Brønsted Acid-Controlled [3 + 2] Coupling Reaction of Quinone Monoacetals with Alkene Nucleophiles: A Catalytic System of Perfluorinated Acids and Hydrogen Bond Donor for the Construction of Benzofurans | The Journal of Organic Chemistry Brønsted Acid-Controlled [3 + 2] Coupling Reaction of Quinone Monoacetals with Alkene Nucleophiles: A Catalytic System of Perfluorinated Acids and Hydrogen Bond Donor for the Construction of Benzofurans | The Journal of Organic Chemistry](https://pubs.acs.org/cms/10.1021/jo400613z/asset/images/medium/jo-2013-00613z_0003.gif)
Brønsted Acid-Controlled [3 + 2] Coupling Reaction of Quinone Monoacetals with Alkene Nucleophiles: A Catalytic System of Perfluorinated Acids and Hydrogen Bond Donor for the Construction of Benzofurans | The Journal of Organic Chemistry



