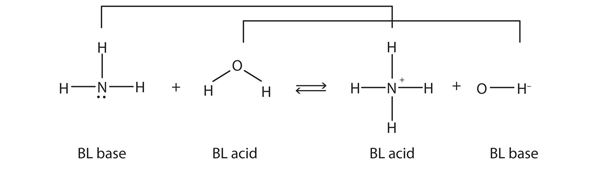
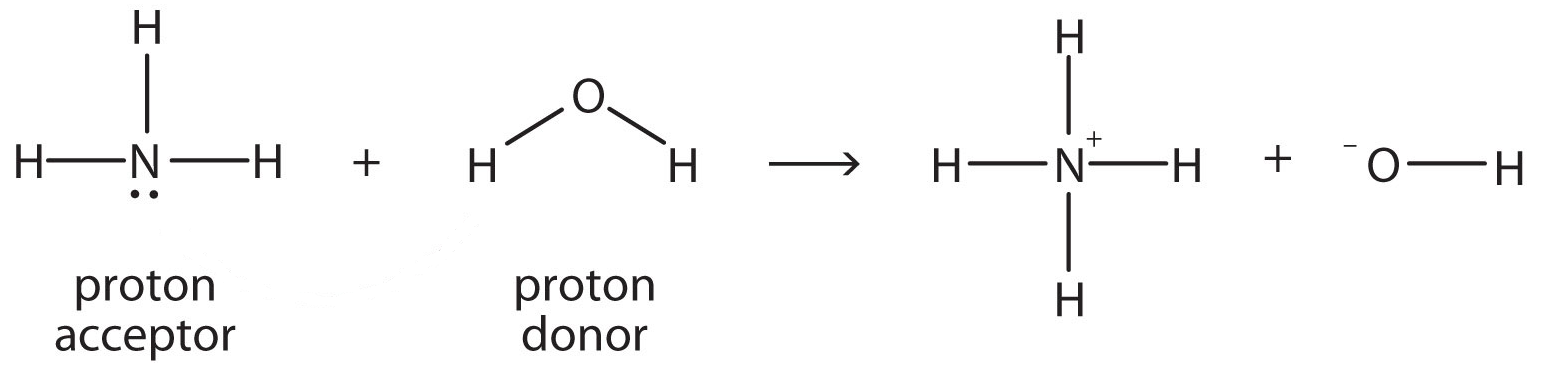
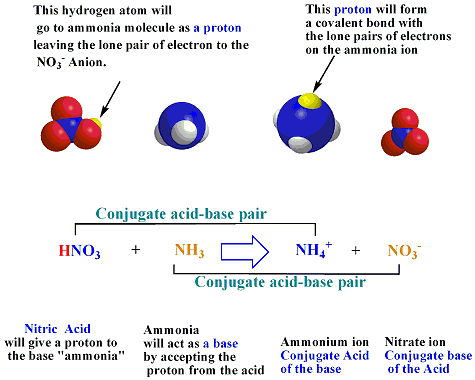
An Alternate Proton Acceptor for Excited-State Proton Transfer in Green Fluorescent Protein: Rewiring GFP | Journal of the American Chemical Society

Proton acceptor (A) and proton donor groups (D) of atorvastatin (a),... | Download Scientific Diagram

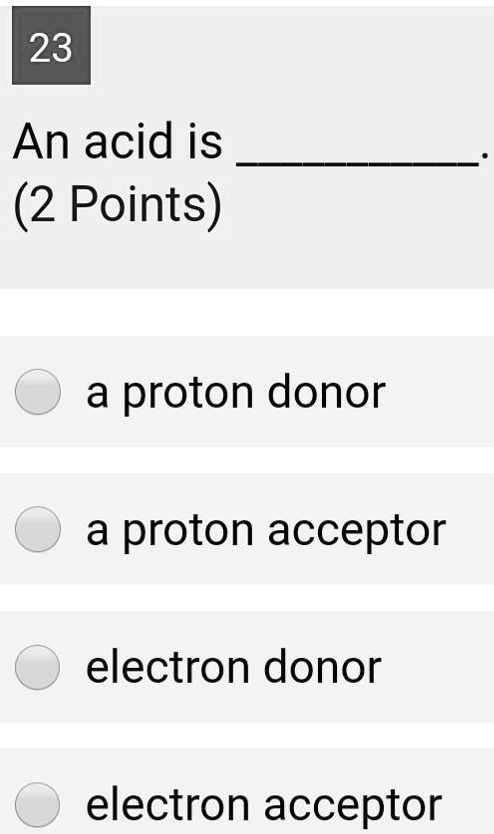
Recap – Last Lecture An acid is a proton donor A base is a proton acceptor A conjugate pair differ by H + Strong A/B is completely dissociated Weak A/B. - ppt

/chapter3/pages19and20/page19and20_files/lewisbronsted.png)